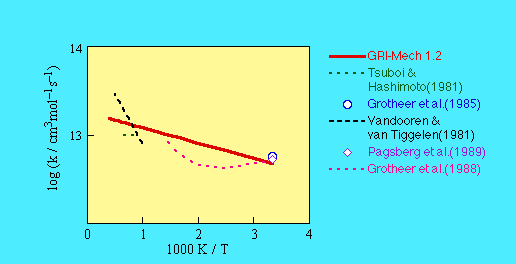
The GRI-Mech 1.2 value was retained for version 3.0
______________________________________________________________________
Temp delta-S delta-H kf kr Keq
(K) (cal/mol K) (kcal/mol) ----(mol,cm3,s)-----
______________________________________________________________________
300 -.3 -19.5 3.98E+12 3.09E-02 1.29E+14
500 -1.2 -19.8 7.28E+12 2.93E+04 2.49E+08
1000 -2.0 -20.4 1.14E+13 1.12E+09 1.02E+04
1500 -2.2 -20.6 1.33E+13 4.04E+10 3.29E+02
2000 -2.2 -20.6 1.44E+13 2.45E+11 5.85E+01
2500 -2.2 -20.5 1.50E+13 7.23E+11 2.08E+01
3000 -2.1 -20.4 1.55E+13 1.48E+12 1.04E+01
_____________________________________________________________________