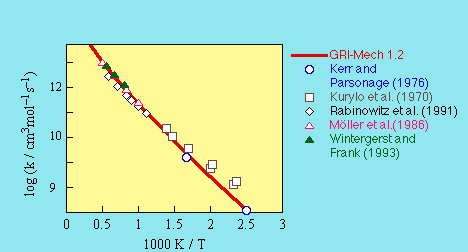
The GRI-Mech 1.2 value was retained for version 3.0
______________________________________________________________________
Temp delta-S delta-H kf kr Keq
(K) (cal/mol K) (kcal/mol) ----(mol,cm3,s)-----
______________________________________________________________________
300 5.7 .8 8.62E+04 2.05E+04 4.20E+00
500 6.8 1.3 2.84E+08 3.36E+07 8.46E+00
1000 7.0 1.3 2.04E+11 1.18E+10 1.73E+01
1500 6.2 .3 2.43E+12 1.20E+11 2.02E+01
2000 5.3 -1.1 9.61E+12 4.88E+11 1.97E+01
2500 4.6 -2.9 2.38E+13 1.33E+12 1.79E+01
3000 3.9 -4.8 4.60E+13 2.92E+12 1.57E+01
______________________________________________________________________