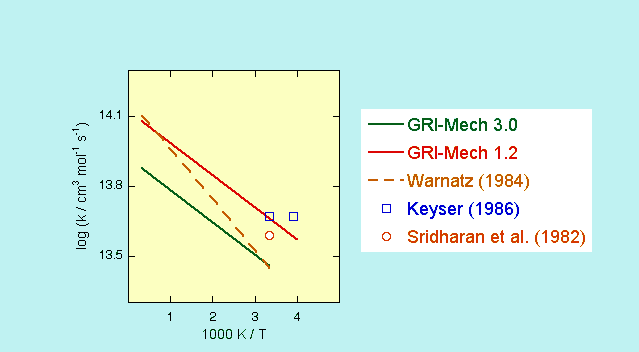
The GRI-Mech 1.2 value was multiplied by a factor of 0.62 for version 3.0
______________________________________________________________________
Temp DS DH kf kr Keq
(K) (cal/mol K) (kcal/mol) ----- (mol,cm3,s) -----
______________________________________________________________________
300 5.7 -36.3 2.90E+13 6.11E-15 4.74E+27
500 5.8 -36.2 4.43E+13 3.41E-04 1.30E+17
1000 5.0 -36.8 6.10E+13 4.28E+04 1.43E+09
1500 4.4 -37.7 6.79E+13 2.44E+07 2.78E+06
2000 3.9 -38.5 7.16E+13 6.27E+08 1.14E+05
2500 3.5 -39.4 7.39E+13 4.59E+09 1.61E+04
3000 3.2 -40.2 7.55E+13 1.78E+10 4.24E+03
______________________________________________________________________
[ Rate coefficient format | Chemkin file | Thermo data | Main menu ]