CH + N2 -> HCN + N
k = 2.86E+08 T^1.1 exp(-20400 cal/mol /RT) cm3/mol s
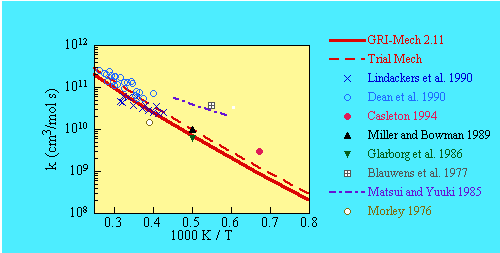
- SOURCE:
-
A preliminary version of an RRKM fit by Rodgers and Smith(1996) to the data of
Dean et al (1990) and Lindackers et al (1990).
- COMMENTS:
-
Served as an optimization variable in GRI-Mech 2.1 release.
The rate coefficient was decreased by a factor of 1.41.
- REFERENCES:
- Rodgers and Smith (1996)
- Rodgers, A.S., and Smith, G.P. (1996) Chem. Phys. Lett. (in press).
- Casleton (1994)
- Casleton, K.H. (1994) private communication.
- Dean et al. (1990)
- Dean, A.J., Hanson, R. K., and Bowman, C.T. (1990) 23rd Symposium
(International) on Combustion, p. 259.
- Lindackers et al. (1990)
- Lindackers, D., Burmeister, M., and Roth, P. (1990)
23rd Symposium (International) on Combustion, p. 251.
- Miller and Bowman (1989)
- Miller, J. A. and Bowman, C. T. (1989)
Prog. Energy Combust. Sci. 15, 287.
- Glarborg et al. (1986)
- Glarborg, P., Miller, J. A., and Kee, R. J.
(1986) Combust. Flame 65, 177.
- Matsui and Yuuki (1985)
- Matsui, Y. and Yuuki, A. (1985)
Jap. J. Appl. Phys. 86, 598.
- Blauwens et al. (1977)
- Blauwens, J., Smets, B., and Peeters, J. (1977)
16th Symposium (International) on Combustion, pp. 1055-1062.
- Morley (1976)
- Morley, G. (1976) Combust. Flame 27, 189.
______________________________________________________________________
Temp delta-S delta-H kf kr Keq
(K) (cal/mol K) (kcal/mol) ----(mol,cm3,s)-----
______________________________________________________________________
300 -4.6 2.5 2.09E-04 1.36E-01 1.53E-03
500 -4.5 2.5 3.23E+02 4.00E+04 8.06E-03
1000 -3.6 3.2 1.99E+07 6.15E+08 3.23E-02
1500 -3.1 3.9 9.50E+08 1.64E+10 5.79E-02
2000 -2.7 4.5 7.22E+09 8.80E+10 8.20E-02
2500 -2.5 5.0 2.57E+10 2.48E+11 1.04E-01
3000 -2.3 5.4 6.24E+10 5.05E+11 1.24E-01
______________________________________________________________________
[ Rate coefficient format |
Chemkin file |
Thermo data |
Main menu ]
