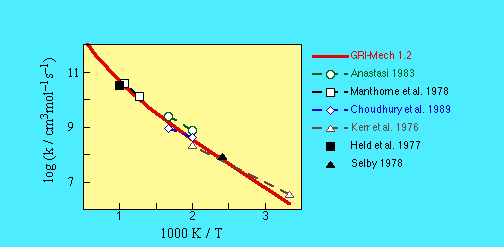
CH3 + CH2O -> HCO + CH4
k = 3.32E03 T^2.81 exp(-5860 cal/mol /RT) cm3/mol s
SOURCE:- This is 0.6 of the expression given by
Tsang and Hampson (1986), as needed to fit the data reported
by Kerr and Parsonage (1976), Anastasi (1983), Choudhury et al. (1989),
and Choudhury and Sanders (1989).
REFERENCES:
- Tsang, W., and Hampson, R.F. (1986) J. Phys. Chem. Ref. Data 15, 1087.
- Anastasi, C. (1983) J. Chem. Soc. Farad. Trans 1 79, 749.
- Manthorne, K.C., and Pacey, P.D. (1978) Can. J. Chem. 56, 1307.
- Choudhury, T.K., Sanders, W.A., and Lin, M.C. (1989)
J. Phys. Chem. 93, 5143.
- Choudhury, T.K., and Sanders, W.A. (1989)
J. Chem. Soc. Farad. Trans. 2 85, 801.
- Held, A.M., Manthorne, K.C., Pacey, P.D., and Reinholdt, H.P.
(1977) Can. J. Chem. 55, 4128.
- Kerr, J.A., and Parsonage, M.J. (1976)
Evaluated Kinetic Data on Gas Phase Hydrogen Transfer Reactions of
Methyl Radicals, Butterworths, London.
- Selby, K.R. (1978) Ph D thesis, York University.
______________________________________________________________________
Temp delta-S delta-H kf kr Keq
(K) (cal/mol K) (kcal/mol) ----(mol,cm3,s)-----
______________________________________________________________________
300 -.5 -17.0 1.63E+06 9.39E-07 1.74E+12
500 -1.0 -17.1 3.50E+08 1.84E+01 1.90E+07
1000 -1.2 -17.3 4.68E+10 1.44E+07 3.25E+03
1500 -1.0 -17.0 3.91E+11 2.16E+09 1.81E+02
2000 -.7 -16.4 1.43E+12 3.23E+10 4.44E+01
2500 -.3 -15.5 3.61E+12 1.82E+11 1.98E+01
3000 .1 -14.4 7.33E+12 6.12E+11 1.20E+01
_____________________________________________________________________
[ Rate coefficient format |
Chemkin file |
Thermo data |
Main menu ]
