H + CH4 -> CH3 + H2
k = 6.60E+08 T^1.62 exp(-10840 cal/mol /RT) cm3/mol s
- SOURCE:
- Transition-state-theory
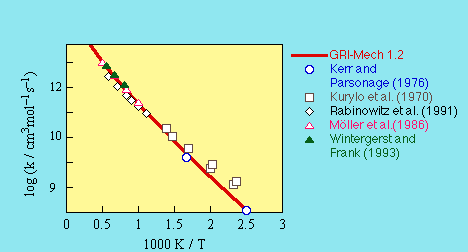
evaluation of Rabinowitz et al. (1991),
Kurylo et al. (1970), Moller et al.(1986), and Kerr and Parsonage (1976).
- COMMENTS:
-
Served as an optimization variable in the GRI-Mech 1.1, 1.2, and 2.1 releases. The
rate coefficient was not changed.
- REFERENCES:
- Rabinowitz et al. (1991)
- Rabinowitz, M.J., Sutherland, J.W., Patterson, P.M., and
Klemm, R.B. (1991) J. Phys. Chem. 95, 674.
- Kurylo et al. (1970)
- Kurylo, M.J., Hollinden, G.A., and Timmons, R.B.
(1970) J. Chem. Phys. 52, 1773.
- Moller et al.(1986)
- Moller, W., Mozzhukhin, E., and Wagner, H.Gg. (1986)
Ber. Bunsenges. Phys. Chem. 90, 854.
- Kerr and Parsonage (1976)
- Kerr, J.A., and Parsonage, M.J. (1976)
Evaluated Kinetic Data on Gas Phase Hydrogen Transfer Reactions
of Methyl Radicals, Butterworths, London.
- Wintergerst and Frank (1993)
- Wintergerst, K., and Frank, P. (1993) to be published.
______________________________________________________________________
Temp delta-S delta-H kf kr Keq
(K) (cal/mol K) (kcal/mol) ----(mol,cm3,s)-----
______________________________________________________________________
300 5.7 .8 8.62E+04 2.05E+04 4.20E+00
500 6.8 1.3 2.84E+08 3.36E+07 8.46E+00
1000 7.0 1.3 2.04E+11 1.18E+10 1.73E+01
1500 6.2 .3 2.43E+12 1.20E+11 2.02E+01
2000 5.3 -1.1 9.61E+12 4.88E+11 1.97E+01
2500 4.6 -2.9 2.38E+13 1.33E+12 1.79E+01
3000 3.9 -4.8 4.60E+13 2.92E+12 1.57E+01
______________________________________________________________________
[ Rate coefficient format |
Chemkin file |
Thermo data |
Main menu ]
