OH + C2H4 -> C2H3 + H2O
k = 3.60E06 T^2 exp(-2500 cal/mol /RT) cm3/mol s
- SOURCE:
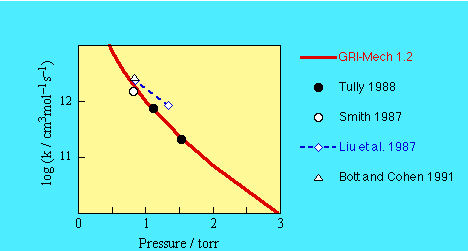
- Tully (1988). Fit to T^2 dependence.
REFERENCES:
- Tully (1988)
- Tully, F.P. (1988) Chem. Phys. Lett. 143, 510.
- Smith (1987)
- Smith, G.P. (1987) Int. J. Chem. Kinet. 19, 269.
- Liu et al. (1987)
- Liu, A.D., Mulac, W.A., and Jordan, C.D. (1987)
Int. J. Chem. Kinet. 19, 25.
- Bott and Cohen (1991)
- Bott, J.F., and Cohen, N. (1991)
Int. J. Chem. Kinet. 23, 1075.
______________________________________________________________________
Temp delta-S delta-H kf kr Keq
(K) (cal/mol K) (kcal/mol) ----(mol,cm3,s)-----
______________________________________________________________________
300 4.7 -8.1 4.89E+09 5.68E+02 8.61E+06
500 5.0 -8.0 7.27E+10 1.87E+06 3.88E+04
1000 4.7 -8.2 1.02E+12 1.51E+09 6.77E+02
1500 4.3 -8.7 3.50E+12 2.13E+10 1.64E+02
2000 4.0 -9.2 7.68E+12 9.90E+10 7.75E+01
2500 3.8 -9.7 1.36E+13 2.82E+11 4.82E+01
3000 3.7 -10.1 2.13E+13 6.16E+11 3.46E+01
_____________________________________________________________________
[ Rate coefficient format |
Chemkin file |
Thermo data |
Main menu ]
