CH + H2 -> H + CH2
k = 1.11E08 T^1.79 exp(-1670 cal/mol /RT) cm3/mol s
SOURCE:
- Zabarnick, S., Fleming, J.W., and Lin, M.C. (1986)
J. Chem. Phys. 85, 4375.
- Becker, K.H., Kurtenbach, R., and Wiesen, P. (1991)
J. Phys. Chem. 95, 2390.
- Bohland, T., Temps, F., and Wagner, H. Gg. (1987)
J. Phys. Chem. 91, 1205.
- McLroy, A., and Tully, F.P. (1993)
J. Chem. Phys. 99, 3597.
-
COMMENTS:
-
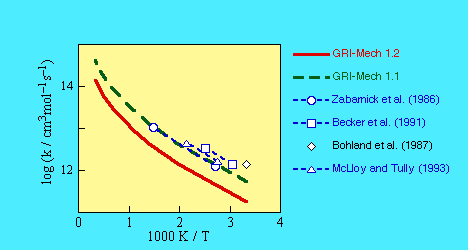
Served as an optimization variable in GRI-Mech 1.2 and 2.1 releases.
As a result of the 1.2 optimization round, the preexponential factor was decreased
by a factor of 3 from the initial fit (see figure below).
______________________________________________________________________
Temp delta-S delta-H kf kr Keq
(K) (cal/mol K) (kcal/mol) ----(mol,cm3,s)-----
______________________________________________________________________
300 -1.1 3.1 1.83E+11 5.82E+13 3.15E-03
500 -1.2 3.1 1.40E+12 5.66E+13 2.47E-02
1000 -1.0 3.3 1.12E+13 9.47E+13 1.19E-01
1500 -.7 3.6 3.07E+13 1.46E+14 2.11E-01
2000 -.6 3.8 5.91E+13 2.06E+14 2.87E-01
2500 -.6 3.8 9.59E+13 2.76E+14 3.48E-01
3000 -.7 3.6 1.41E+14 3.57E+14 3.94E-01
_____________________________________________________________________
[ Rate coefficient format |
Chemkin file |
Thermo data |
Main menu ]
