CH3 + O2 -> O + CH3O
k = 2.675E13 exp(-28800 cal/mol /RT) cm3/mol s
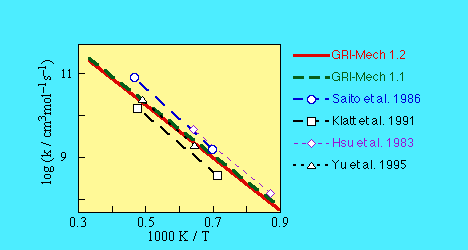
- SOURCE:
- The initial rate coefficient was a
consensus expression based upon results reported by
-
- Saito, K., Ito, R., Kakumoto, T., and Imamura, A. (1986)
J. Phys. Chem. 90, 1422.
- Klatt, M., Rohrig, M., and Wagner, H.Gg. (1991)
Ber. Bunsenges. Phys. Chem. 95, 1163.
- Hsu, D.S.Y., Shaub, W.M., Creamer, T., Gutman, D., and Lin, M.C. (1983)
Ber. Bunsenges. Phys. Chem. 87, 909.
- Yu, C.-L., Wang, C., and Frenklach, M. (1995)
J. Phys. Chem. 99, 14377.
-
The rate coefficient then served as an optimization variable in
GRI-Mech 1.1, 1.2, and 2.1 releases.
As a result of the 1.2 optimization round, the preexponential factor was lowered
by 11% from the initially assigned expression, and remained the same after the
2.1 optimization round.
______________________________________________________________________
Temp delta-S delta-H kf kr Keq
(K) (cal/mol K) (kcal/mol) ----(mol,cm3,s)-----
______________________________________________________________________
300 -2.3 28.3 2.80E-08 3.93E+13 7.11E-22
500 -3.0 28.1 6.90E+00 5.84E+13 1.18E-13
1000 -2.8 28.3 1.36E+07 8.34E+13 1.63E-07
1500 -2.2 29.0 1.70E+09 8.66E+13 1.97E-05
2000 -1.8 29.7 1.91E+10 8.30E+13 2.30E-04
2500 -1.4 30.6 8.12E+10 7.76E+13 1.05E-03
3000 -1.1 31.3 2.13E+11 7.22E+13 2.96E-03
_____________________________________________________________________
[ Rate coefficient format |
Chemkin file |
Thermo data |
Main menu ]
