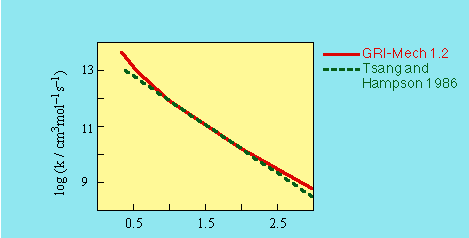
H + H2O2 -> HO2 + H2
k = 1.21E+07 T^2 exp(-5200 cal/mol /RT) cm3/mol s
SOURCE:- Fit to the data reported in Tsang and Hampson (1986)
to a T^2 temperature dependence as expected from transition state theory.
REFERENCES:- Tsang, W., and Hampson, R.F. (1986)
J. Phys. Chem. Ref. Data 15, 1087.
______________________________________________________________________
Temp delta-S delta-H kf kr Keq
(K) (cal/mol K) (kcal/mol) ----(mol,cm3,s)-----
______________________________________________________________________
300 2.5 -16.6 1.77E+08 3.85E-05 4.60E+12
500 2.4 -16.7 1.61E+10 2.46E+02 6.56E+07
1000 1.8 -17.2 8.84E+11 6.39E+07 1.38E+04
1500 1.2 -17.8 4.76E+12 6.42E+09 7.41E+02
2000 .9 -18.5 1.31E+13 8.08E+10 1.62E+02
2500 .7 -18.9 2.66E+13 4.21E+11 6.31E+01
3000 .5 -19.3 4.55E+13 1.37E+12 3.32E+01
______________________________________________________________________
[ Rate coefficient format |
Chemkin file |
Thermo data |
Main menu ]
