H + HO2 -> O + H2O
k = 3.97E+12 exp(-671 cal/mol /RT) cm3/mol s
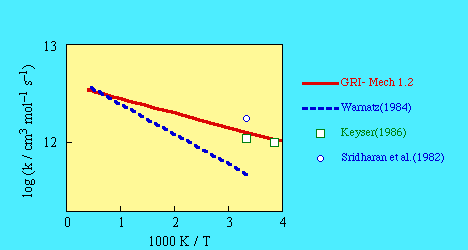
SOURCE:- A least-squares fit to the data from Dixon-Lewis (1987),
Sridharan et al. (1982), and Keyser (1986).
REFERENCES:
- Dixon-Lewis (1987)
- Dixon-Lewis, G. (1987) in Complex Chemical Reactions Systems.
Mathematical Modelling and Simulation, J. Warnatz and W. Jager, Eds.,
Springer-Verlag, Berlin.
- Sridharan et al. (1982)
- Sridharan, W.C., Qiu, L.X. and Kaufman, F. (1982)
J. Phys. Chem. 86, 4569.
- Keyser (1986)
- Keyser, L.F. (1986) J. Phys. Chem. 90, 2994.
- Warnatz (1984)
- Warnatz, J. (1984) in Combustion Chemistry,
W.C. Gardiner, Ed., Springer-Verlag, Chap. 5.
______________________________________________________________________
Temp delta-S delta-H kf kr Keq
(K) (cal/mol K) (kcal/mol) ----(mol,cm3,s)-----
______________________________________________________________________
300 1.4 -53.3 1.29E+12 8.54E-28 1.51E+39
500 1.2 -53.5 2.02E+12 4.79E-12 4.22E+23
1000 .3 -54.1 2.83E+12 3.64E+00 7.78E+11
1500 -.3 -54.8 3.17E+12 3.76E+04 8.43E+07
2000 -.6 -55.3 3.35E+12 4.02E+06 8.35E+05
2500 -.8 -55.8 3.47E+12 6.79E+07 5.11E+04
3000 -.9 -56.2 3.55E+12 4.54E+08 7.82E+03
______________________________________________________________________
[ Rate coefficient format |
Chemkin file |
Thermo data |
Main menu ]
