O + C2H4 -> CH3 + HCO
k = 1.92E+07 T^1.83 exp(-220 cal/mol /RT) cm3/mol s
- SOURCE:
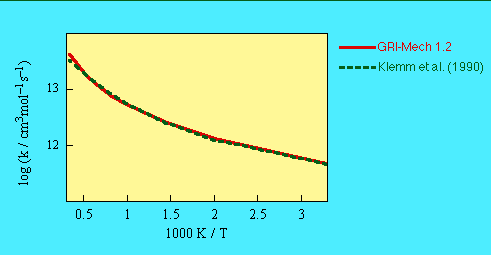
- We assumed the reaction products to be CH3 + HCO and fitted the
data reported by Klemm et al. (1990).
- COMMENTS:Klemm et al. (1990) provided a dual-exponential fit to their
results. Baulch et al. (1992) chose a procedure similar to ours, omitted a small
activation energy term in the final fit, and recommended expressions that yield 60% for the
products given here and 35% CH2CHO + H. We did not include the latter channel in
order to avoid having to add CH2CHO to the set of species. At high temperatures
it is likely that direct H abstraction also contributes.
REFERENCES
- Klemm et al. (1990)
- Klemm, R.B., Sutherland, J.W., Wickramaaratchi, M.A.,
and Yarwood, G.
(1990) J. Phys. Chem. 94, 3354.
- Baulch et al. (1992)
- Baulch, D.L., Cobos, C.J., Cox, R.A., Esser, C.,
Frank, P., Just, Th.,
Kerr, J.A., Pilling, M.J., Troe, J., Walker, R.W., and Warnatz, J. J.
(1992) Phys. Chem. Ref. Data 21, 411.
______________________________________________________________________
Temp delta-S delta-H kf kr Keq
(K) (cal/mol K) (kcal/mol) ----(mol,cm3,s)-----
______________________________________________________________________
300 9.1 -27.0 4.53E+11 1.09E-10 4.15E+21
500 9.6 -26.8 1.34E+12 2.13E-02 6.28E+13
1000 8.9 -27.4 5.31E+12 6.44E+04 8.24E+07
1500 8.0 -28.4 1.16E+13 1.49E+07 7.77E+05
2000 7.3 -29.7 2.00E+13 2.93E+08 6.82E+04
2500 6.7 -31.0 3.04E+13 2.05E+09 1.48E+04
3000 6.2 -32.4 4.27E+13 8.33E+09 5.12E+03
______________________________________________________________________
[ Rate coefficient format |
Chemkin file |
Thermo data |
Main menu ]
