O + CH2O -> OH + HCO
k = 3.90E+13 exp(-3540 cal/mol /RT) cm3/mol s
SOURCE:
A least-squares fit to survey of data in NIST database:
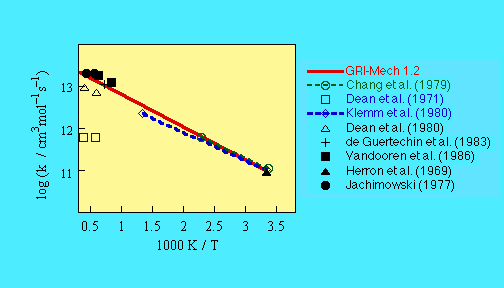
- Chang, J.S., and Barker, J.R. (1979) J. Phys. Chem. 83, 3059.
- Dean, A.M., and Kistiakowsky, G.B. (1971) J. Chem. Phys. 54, 1718.
- Klemm, R.B., Skolnik, E.G., and Michael, J.V. (1980) J. Chem. Phys.
72, 1256.
- de Guertechin, L.O., Vandooren, J., and Van Tiggelen P.J. (1983)
Bull. Soc. Chim. Belg. 92, 663.
- Dean, A.M., Johnson, R.L., and Steiner, D.C. (1980) Combust. Flame
37, 41.
- Vandooren, J., Oldenhove deGuertechin, L., and VanTiggelen, P.J.
(1986) Combust. Flame 64, 127.
- Herron, J.T., and Penzhorn, R.D. (1969) J. Phys. Chem. 73, 191.
- Jachimowski, C.J. (1977) Combust. Flame 29, 55.
______________________________________________________________________
Temp delta-S delta-H kf kr Keq
(K) (cal/mol K) (kcal/mol) ----(mol,cm3,s)-----
______________________________________________________________________
300 6.8 -14.2 1.03E+11 1.66E-01 6.19E+11
500 7.4 -13.9 1.11E+12 2.20E+04 5.03E+07
1000 7.3 -14.0 6.57E+12 1.41E+08 4.65E+04
1500 6.8 -14.7 1.19E+13 2.81E+09 4.23E+03
2000 6.3 -15.5 1.60E+13 1.34E+10 1.20E+03
2500 6.0 -16.3 1.91E+13 3.55E+10 5.39E+02
3000 5.7 -17.1 2.15E+13 6.99E+10 3.08E+02
______________________________________________________________________
[ Rate coefficient format |
Chemkin file |
Thermo data |
Main menu ]
