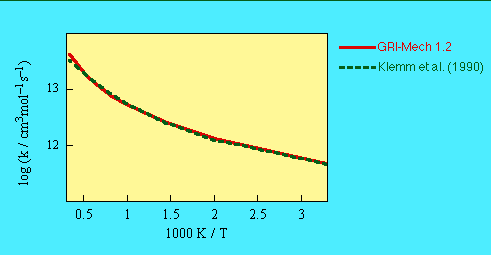
Total rate constant for k(25) + k(285) is the same as the GRI-Mech 1.2 value.
______________________________________________________________ Temp DS DH kf kr Keq (K) (cal/mol K) (kcal/mol) ----- (mol,cm3,s) ----- ______________________________________________________________ 298 0.5 -14.0 1.56E+11 7.05E+00 2.21E+10 300 0.6 -13.9 1.58E+11 8.26E+00 1.91E+10 400 1.3 -13.7 2.94E+11 5.06E+03 5.80E+07 500 1.8 -13.5 4.67E+11 2.46E+05 1.89E+06 1000 2.7 -12.8 1.85E+12 7.45E+08 2.49E+03 1500 3.0 -12.5 4.04E+12 1.36E+10 2.97E+02 2000 3.0 -12.5 6.96E+12 6.70E+10 1.04E+02 2500 2.9 -12.7 1.06E+13 1.92E+11 5.52E+01 3000 2.8 -13.0 1.49E+13 4.15E+11 3.59E+01 ______________________________________________________________
[ Rate coefficient format | Chemkin file | Thermo data | Main menu ]